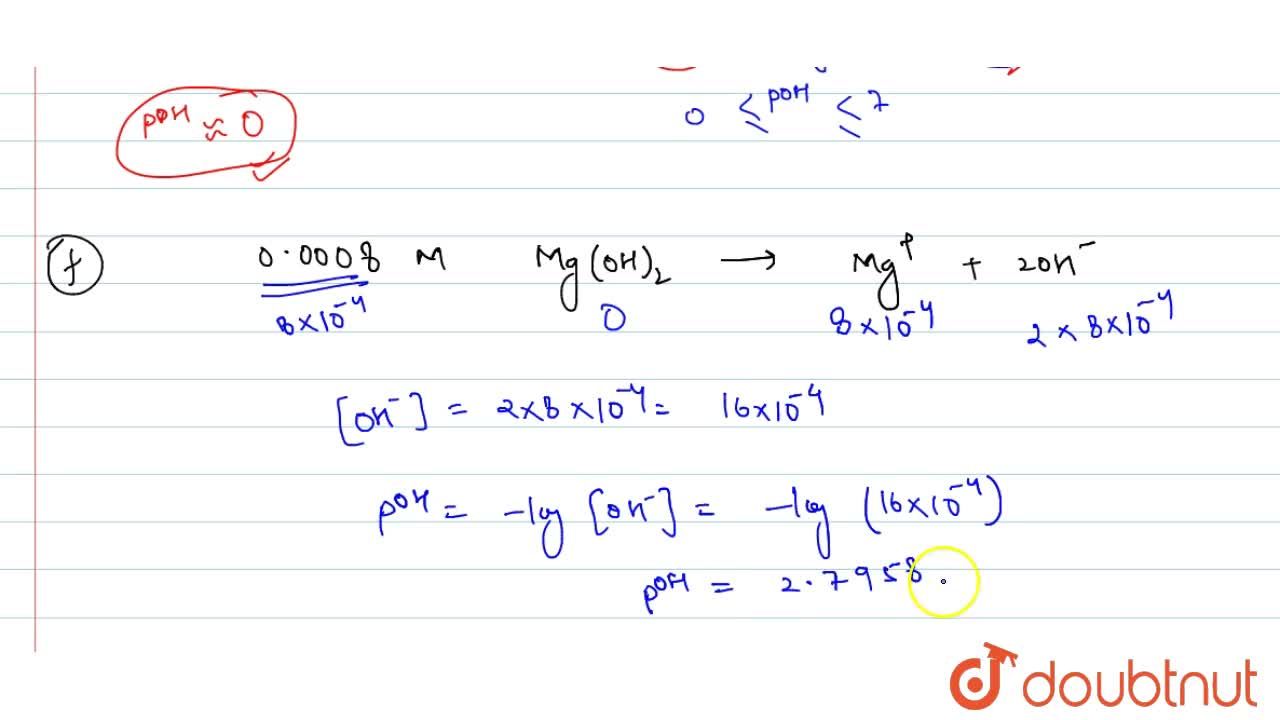
Calculate pH of the following solutions: (i) 0.001M HNO3 (ii) 0.005M H2SO4 (iii) 0.01M KOH (iv) 10^-8M NaOH (v) 0.0008M Ba (OH) 2

Calculate pH of the following solutions: (i) 0.001M HNO3 (ii) 0.005M H2SO4 (iii) 0.01M KOH (iv) 10^-8M NaOH (v) 0.0008M Ba (OH) 2

The ionisation constant of formic acid HCO2H is 1.8× 10^-4.what is percentage ionisation of a 0.001M solution of this acid? | EduRev NEET Question

Calculate pH for: (a) `0.001 NaOH`, (b) `0.01N Ca(OH)_(2)`, (c ) `0.01M Ca(OH)_(2)`, (d) `10^(-8 - YouTube